India joined WTO (World Trade Organization) and became a signatory of the TRIPS (Trade-Related Aspects of Intellectual rights) agreements in the year of 1995. With this, all the signatories were supposed to align their IP rules in conformation with the TRIPS agreement. However, developing countries like India were granted a window period of 10 years (5-compulsory +5 extended) to comply with the rules put forth by the agreement.
Though India had aligned its rule in accordance to TRIPS in the year 2005, still, there are many challenges and issues, that needs to be addressed to maximize the benefits. Thus getting and granting IP rights in India has become a matter of contention since 2005 and various stakeholders are interested in knowing India address these issues.
This article is an attempt to underline those challenges and issues that India is facing in offering IP rights to companies in Indian jurisdiction. Though, there are many challenges we will list only the top 6, that are of utmost importance.
Intellectual Property Rights (India): Top 6 Challenges
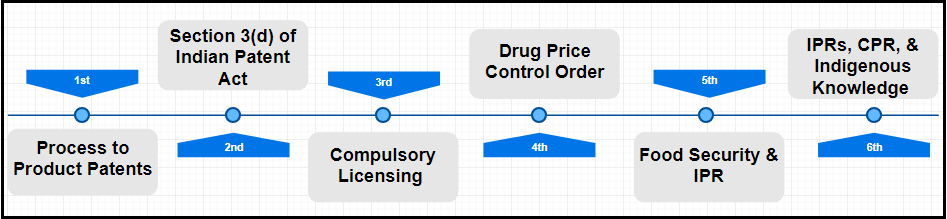
1# From Process to Product Patents- One of the binding point in TRIPS agreement is that all member countries are required to shift their patent regime from “Process Patent” to “Product Patent.” The fundamental difference between a Process Patent regime and a Product Patent regime lies in the fact that the former protects for processes only while the latter products. It becomes a contentious issue when it comes to getting IP rights on pharmaceuticals and food products.
Unlike developed countries where Capitalist Economic Model is working India has adopted a mixed development model striking a balance between Capitalism and Socialism. This approach was taken to safeguard the interest of ordinary people those are struggling for their basic needs including food and medicines. Developed countries are accusing countries like India and Brazil being protectionist when it comes to granting patents in pharmaceuticals and food sectors.
2# Section 3(d) of the Indian Patent Act– Another challenge that it is facing is the condemnation of section 3(d) of the Indian Patent Act. This section prevents multinational companies evergreening their patents simply by making minor changes. Implementation of 3(d) was exercised in challenging the patent of Novartis Glevac drug. The Court rules that multinational companies can’t evergreen their patents simply by making minor changes in earlier patents and they need to show considerable “Therapeutic Efficiency” to get patent protection in already existing patents.
3# Compulsory licensing- With the provision of compulsory licensing, the Govt of India can compel the owner company or other companies to mass produce some drugs in emergency irrespective of who got the patent. Multinationals are accusing India of being opportunistic in their stand and are asking to abrogate this provision. However, Indian Govt is not willing to cancel this provision to safeguard the interests of mass.
4# Provision of Drug Price Control Order- With this provision companies can’t charge an unfair price for drugs that they are producing. The price has to be justified regarding investments, and if someone plays foul, then the Govt has the right to intervene.
5# Food security and IPR- India is a land of farmers wherein most of the people are engaged in doing farming for their livelihood. In such a country Govt offers many subsidies to farmers. India’s domestic support schemes are generally in the form of “minimum support price” for major agricultural commodities and “input” subsidies provided to farmers in the types of electricity, fertilizers, seeds, etc. However, for complete implementation of TRIPS agreements, these subsidies will have to be reduced or eliminated. Thus, the Indian Government is struggling to create a balance between food security and providing IP rights in India.
6# IPRs, Community property rights, & Indigenous knowledge- Traditional knowledge gives ready-made leads for pharmaceutical companies and then simply come up with the new formulation to show the efficacy of the general traditional understanding. The Indian Govt is bound to protect the rich source of traditional knowledge by not allowing multinationals to get patents on traditional culture. As a defensive mechanism, the Govt has created TKDL (Traditional Knowledge Digital Library) to challenge patenting traditional Indian understanding. Multinationals and developed countries are also opposing this move.
Intellectual Property Rights (India): Neglected Issues
Some of the highlighted issues that are facing negligence regarding implementation, for a long time are:
- Insufficiency of the regulations,
- Lack of awareness and respect for IPRs and access rules, and
- Lack of efficient application/control of these regulations.
Intellectual Property Rights (India): Some Recommendations To Follow
Some of our recommendations that can be followed are:
- Formulate comprehensive IPR Policies for various sectors and Academic Institutions
- Train personnel to manage IPR
- Provide access and training to use Patent information databases
- Create a consortium of IPR professionals to offer professional services for IPR work
Intellectual Property Rights (India): Milestones To Achieve
Though India technically has shifted from a process patent regime to a product patent regime, it is still struggling to balance the interest of companies as well as the masses. The country has technically incorporated the rules of TRIPS but also trying to level the playing field by adding clauses and provisions like compulsory licensing. Some of the milestones still to achieve, are:
- Successful implementation of the TRIPs agreement: The important ones being legal, administrative and institutional reforms, appropriate research investment, and first-rate science and technology capability. Provided the IPR protection is adequate and effective (worldwide), the TRIPs accord can promote innovation, transfer of technology, foreign direct investment, use of genetic resources and environmental protection.
- Creation of patent cell in the ICAR: Having a clear-cut intellectual property policy and promoting patent literacy among its scientists must be the next logical step.
- Foster and reward entrepreneurship: To maximize opportunities patent offices must evolve a regulatory environment conducive to technological innovation.
- Indian must establish a lobby: At the international level, in the WTO, India must lobby for creating a linkage between the Convention on Biological Diversity (CBD) and TRIPs, stating that it is the CBD which must have primacy over the TRIPs and not the other way round.
Getting IP rights in India is a complex process where plenty of clauses and provisions are there those can interfere with the interests of patentees. Thus, it is of utmost importance to invest prudently, foreseeing risks that companies may face. In such a situation it is essential to take help of companies that are experienced in filing patents and protecting IP rights in India. Should you are looking for such a company, Your Patent Team can help you in your journey from ideating to protecting IPs.
Visit our service page, for more information.
Also Read:
India Enters WIPO Digital Access Service System
Software Patents Under Indian Patent Law
What Are The Inventions That Can Be Patented In India?
How to file Patent in India- Requirements | Procedure | Specifications | Forms
Here you can Download our FREE Help Guides: